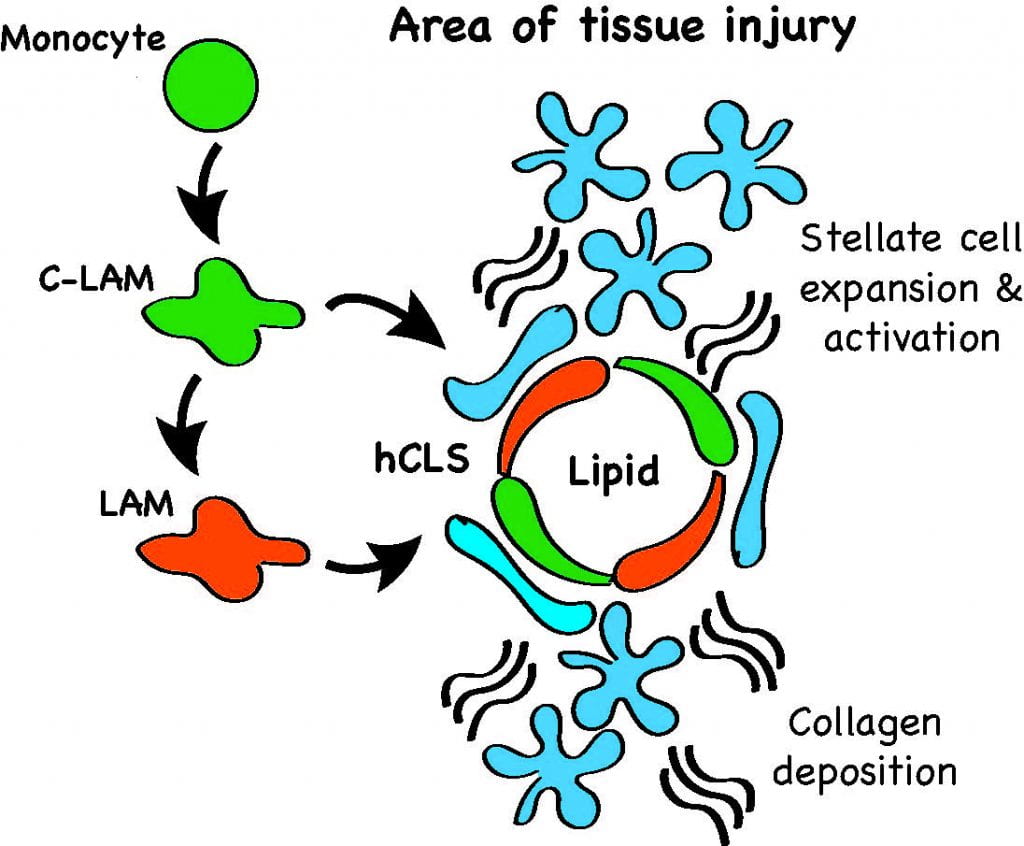
Monocyte-derived macrophages in NASH pathogenesis and Fibrosis:
During the pathogenesis of NASH monocyte-derived macrophages (MdMs) accumulate in the liver and tend to localize in macrophage aggregates referred to as hepatic crown-like structures (hCLS). Using single cell RNA sequencing, flow cytometry, and tissue imaging we have identified two populations of MdMs that express the lipid receptor Trem2. One macrophage subset also expresses Cx3cr1 and Ccr2 and the second subset expresses Cd63, Cd9, and Gpnmb, which are markers previously ascribed to lipid-associated macrophages (LAMs) in obese adipose tissue. As such, these macrophage subsets are referred to as hepatic C-LAMs and LAMs, respectively. C-LAMs and LAMs closely interact with hepatic stellate cells in the hCLS. Moreover, disruption of MdM recruitment prevents hCLS formation and reduces myofibroblast formation but leads to enhanced tissue fibrosis. Thus, macrophages in hCLS appear to have pro-fibrotic and anti-fibrotic characteristics that shape tissue remodeling during NASH. Our data, in conjunction with that from other labs, has shown that hCLS are associated with NASH progression in mice and humans and that these structures appear to regulate the dynamics of tissue remodeling. Ongoing projects seek to understand the molecular crosstalk talk pathways between MdMs and stellate cells, the regulation of matrix breakdown by macrophages, and pathways that lead to macrophage fate decisions.
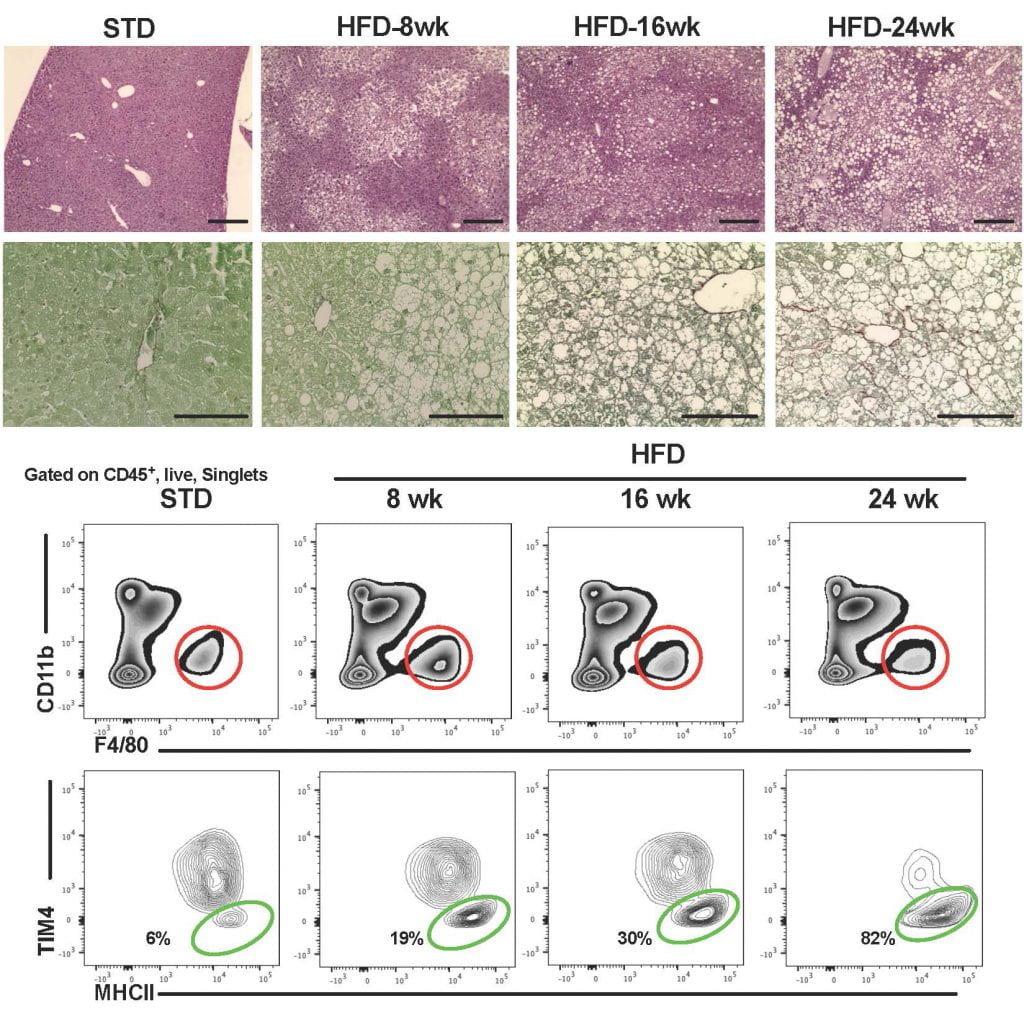
Mechanisms and consequences of resident KC death in NASH:
Resident KCs are embryonically derived macrophages that reside in the liver sinusoids where they play a role in liver homeostasis and phagocytosis of blood borne pathogens and debris. During the progression of NASH KCs undergo cell death and decrease in number. In response to KC loss a subset of monocyte-derived cells differentiate along a KC pathway to become monocyte-derived KCs (Mo-KCs). We are currently focused on addressing several important questions about KC biology in NASH. Specifically, we are investigating: 1) how lysosome dysfunction in KCs contributes to cell dysfunction and death 2) What are the consequences of KC loss on liver filtration function? 3) How do Mo-KCs function in the NASH environment? To address some of these questions were have generated genetic models to alter the expression of molecules involved in lysosome-mediated cell death and inflammation (i.e. NLRP3 or TFEB) specifically in KCs or MdMs.
Unraveling the consequences of right heart failure on liver macrophages and systemic inflammation:
Right heart failure in common in patients with cardiomyopathy or pulmonary hypertension. In response to RV failure central venous pressure rises, placing stress on the liver. Over time, pressure overload in the liver can lead to fibrosis and in severe cases cirrhosis. Moreover, right heart failure in patients with cardiomyopathy is associated with increased systemic inflammation and poor prognosis. However, the mechanisms and consequences of immune cell activation by elevated sinusoidal pressures is poorly understood. Using a combination human samples and mouse models we are investigating the impact of right heart failure on liver immune cells.